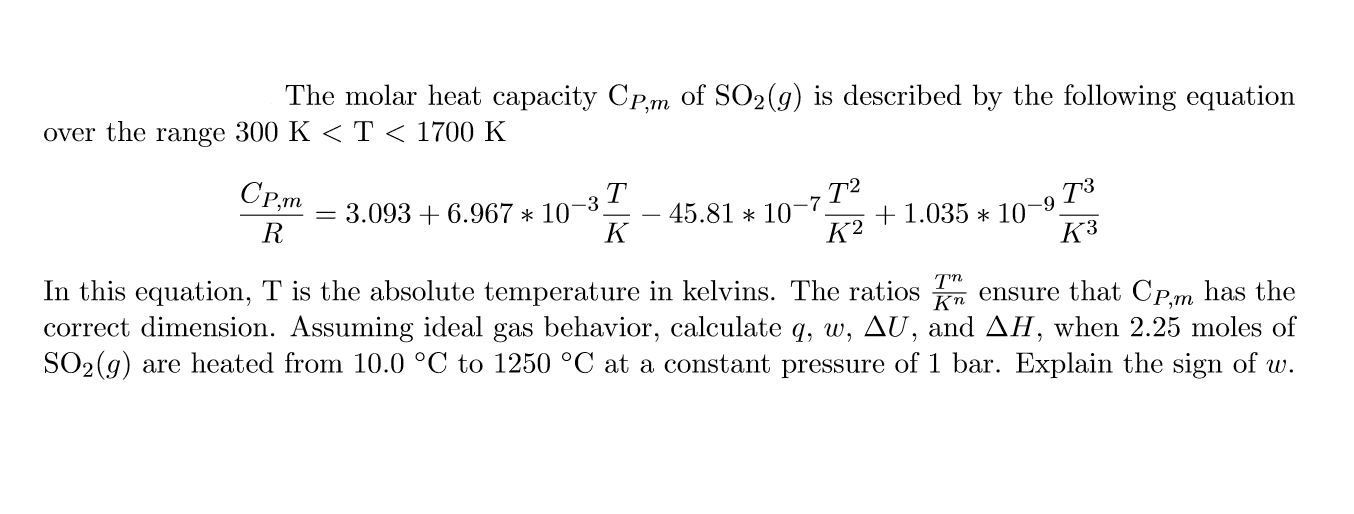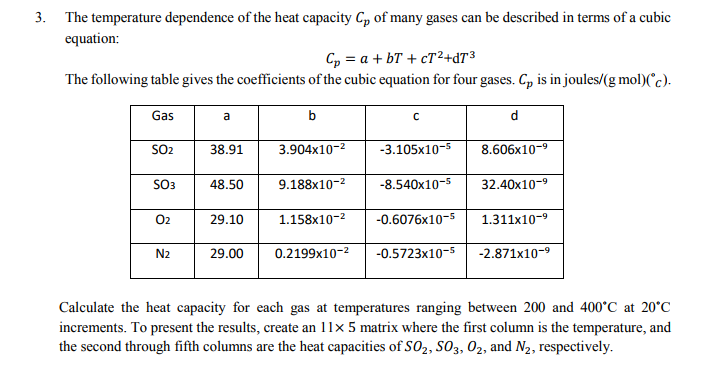
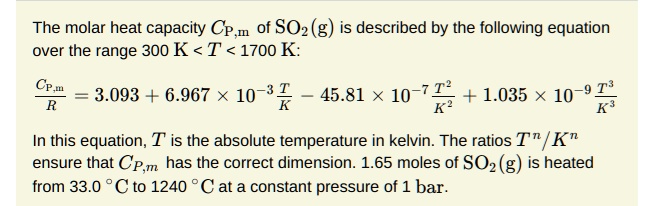
SOLVED: How much heat energy is required to convert 98.1 g of liquid sulfur dioxide, SO2, at 200. K to gaseous SO2 at 263 K? The molar heat of vaporization of SO2

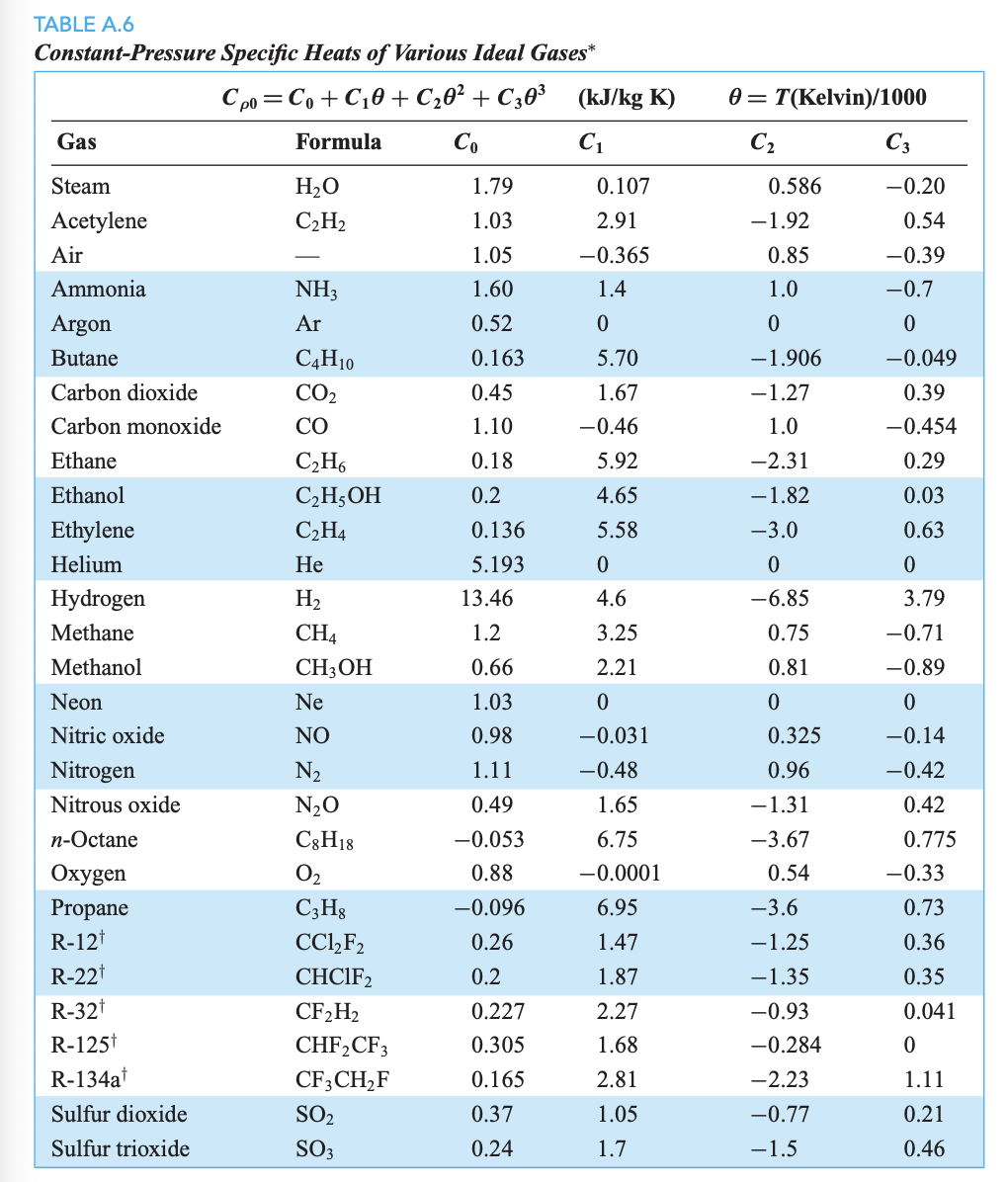
Specific Heat Capacities of Two Functional Ionic Liquids and Two Functional Deep Eutectic Solvents for the Absorption of SO2 | Journal of Chemical & Engineering Data

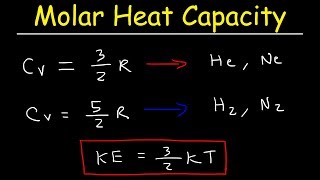
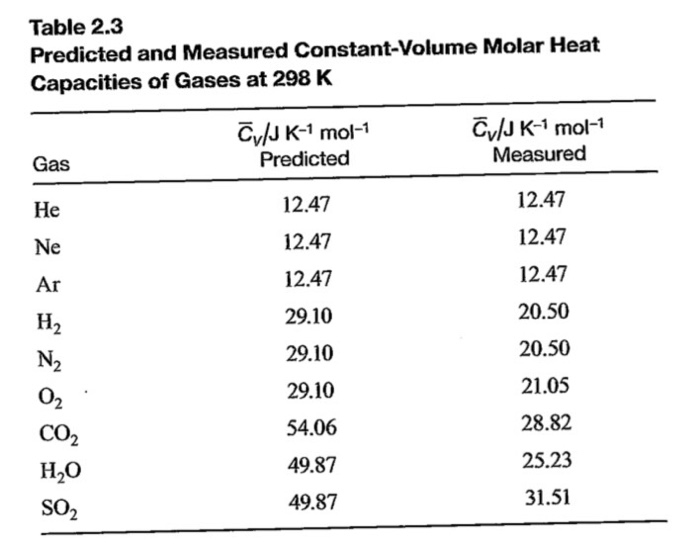
For each polyatomic gas CO2, SO2, H2S compute the value of the molar heat capacity at constant volume, Cv on the assumption that there is no vibrational energy. Compare with the measured
When 0.5 g of sulphur is burnt to SO_2 ; 4.6 kJ of heat is liberated. What is the enthalpy of formation of sulphur dioxide ? (a) 147 kJ (b) +147.2kJ (c) +294.4 kJ(d) 294.4k
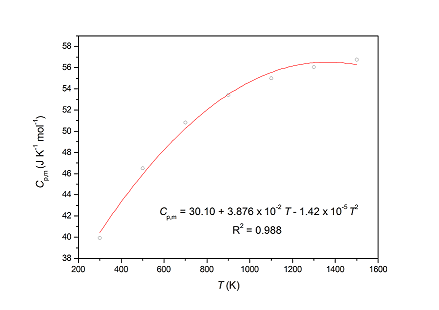
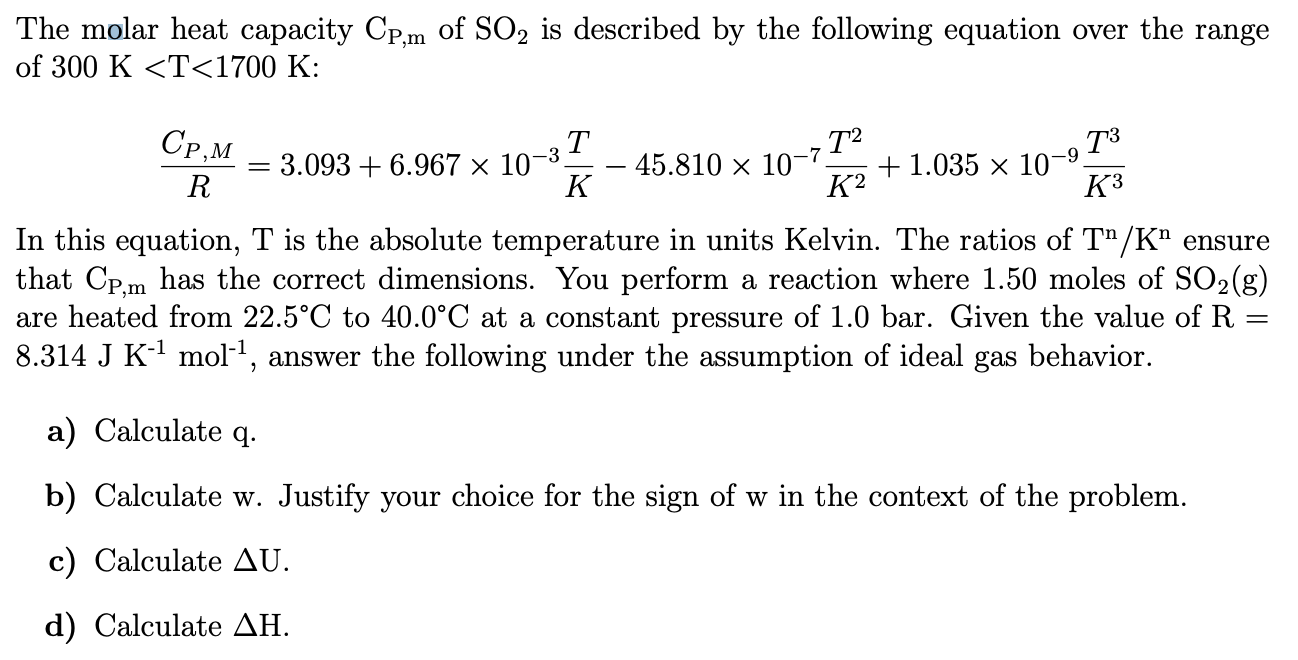
SOLVED: The molar heat capacity Cpm of SO2(g) is described by the following equation over the range 300 K < T < 1700 K: Cpm 3.093 + 6.967 10-3 % 45.81 10-732
SOLVED: How much heat energy is required to convert 97.9 g of liquid sulfur dioxide, SO2, at 198.5 K to gaseous SO2 at 263.1 K if the molar heat of vaporization of
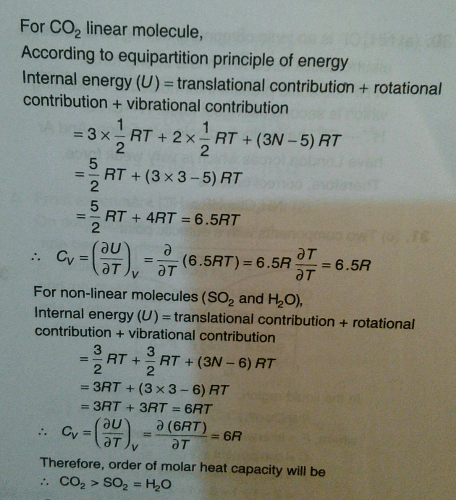
According to the equipartition principle of energy, the molar heat capacity at constant volume for CO2(g), SO2(g) and H2O(g) follows the trends:a)CO2 = SO2 = H2Ob)CO2 < SO2 = H2Oc)CO2 > SO2 =

Specific Heat Capacity of Non-Functional and Functional Ionic Liquids during the Absorption of SO2 | Industrial & Engineering Chemistry Research

The value of heat of formation of SO2 and SO3 are - 398.2 kJ and - 198.2 kJ . The heat of formation of this reaction will be SO2 + 12O2→ SO3

SOLVED: The molar heat capacity Cpm of equation over the SO2(g) is described by the range 300 K<T < 1700 K: following Cpm = 3.093 + 6.967 x 10 3T 45.81 X10